You have already had a tissue biopsy test but the results were incomplete or inconclusive. Our solutions for patients with cancer are enabled by Guardant.
 Fda Approves Blood Tests That Can Help Guide Cancer Treatment National Cancer Institute
Fda Approves Blood Tests That Can Help Guide Cancer Treatment National Cancer Institute
The Guardant360CDx is approved both to provide information on multiple solid tumor biomarkers and to help identify EGFR mutations in patients who will benefit from treatment with TAGRISSO.

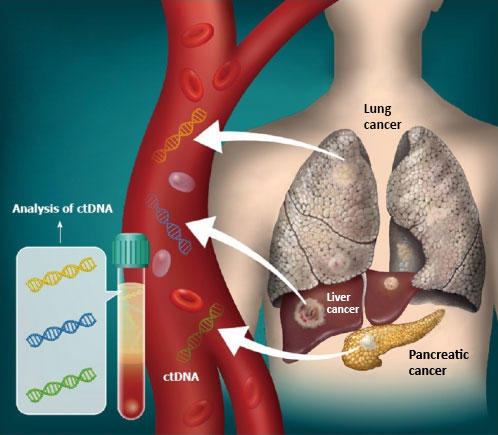
What is guardant360. Guardant360검사는 진행성 고형암을 가진 암환자를 위한 Guardant Health의 혁신적인 액체생검 검사입니다. Besides while median time to results for Guardant360 was 9 days it was 15 days for tissue testing. 이 검사는 종양세포에서 유리된 작은 유전자 조각이 혈류로 방출되어 나온 순환 종양 DNA circulating tumor DNA ctDNA를 이용하여 분석합니다.
You are already on treatment but your cancer has been progressing. Before first-line therapy n81 EGFR ALK MET BRCA1 ROS1 RET ERBB2 or BRAF. It is used by oncologists in leading cancer centers and more than 1000 hospitals worldwide.
미량의 순환 종양 DNA는 디지털 시퀀싱 기술을 사용하여 혈액에서 감지할 수 있습니다. You or a loved one has recently been diagnosed with cancer and have not started treatment. With the use of a single blood test the companys goal is to detect ctDNA through sequencing cell-free DNA and finding mutations and epigenetics changes that occur in cancer cells.
Guardant360 and tissue testing results were concordant in more than 90 of cases. Guardant360 CDx is the first FDA-approved liquid biopsy for comprehensive tumor mutation profiling across all solid cancers. GTR Test ID Help Each Test is a specific orderable test from a particular laboratory and is assigned a unique GTR accession number.
I appreciate your feedback. For targetable alterations. Before first-line therapy n81 EGFR.
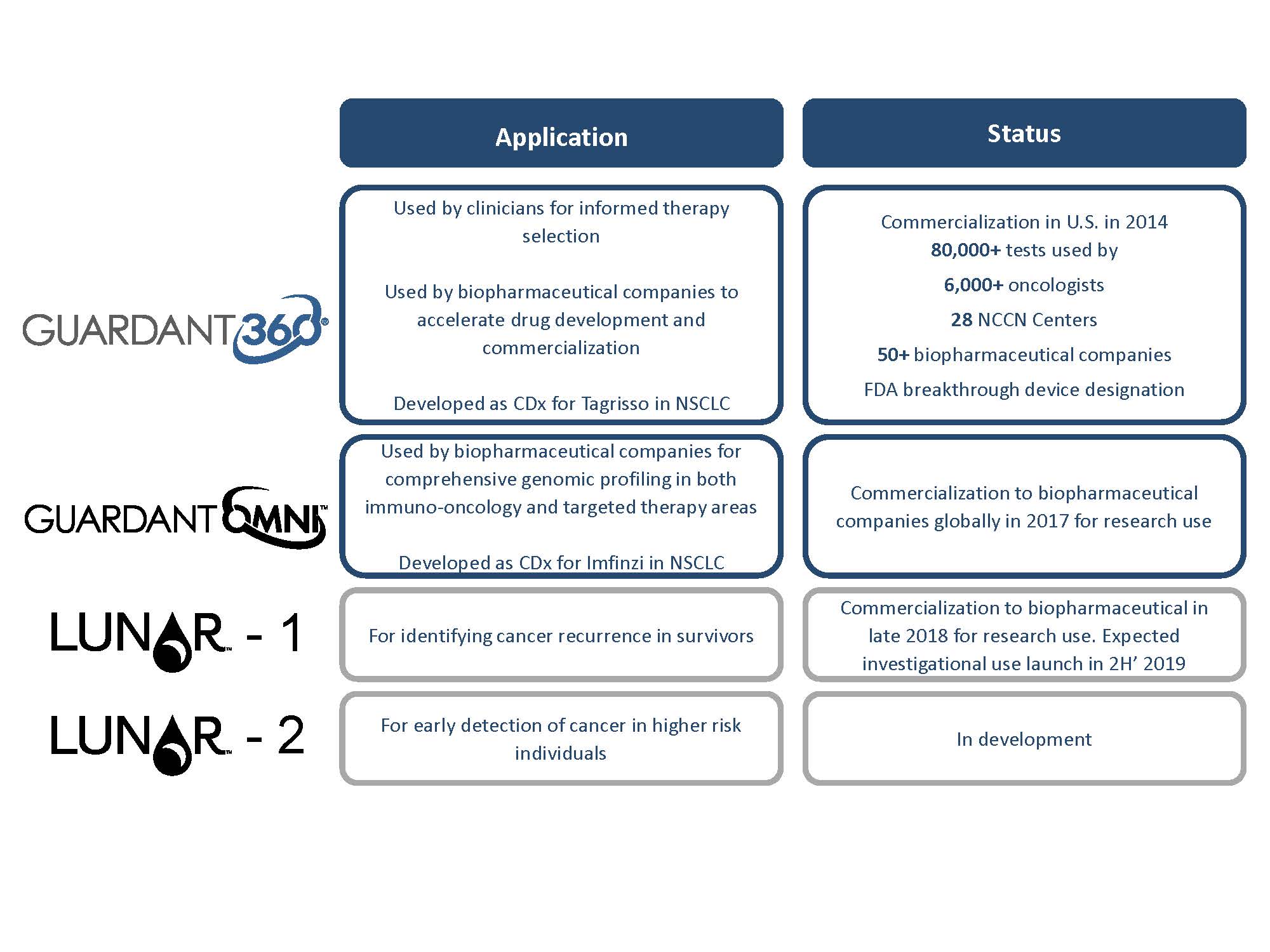
The Guardant360 assay provides comprehensive genomic profiling information that can. The Guardant360 test is also useful to pharmaceutical companies as it is enabling the advancement of new therapies to market faster. 70개 이상의 임상.
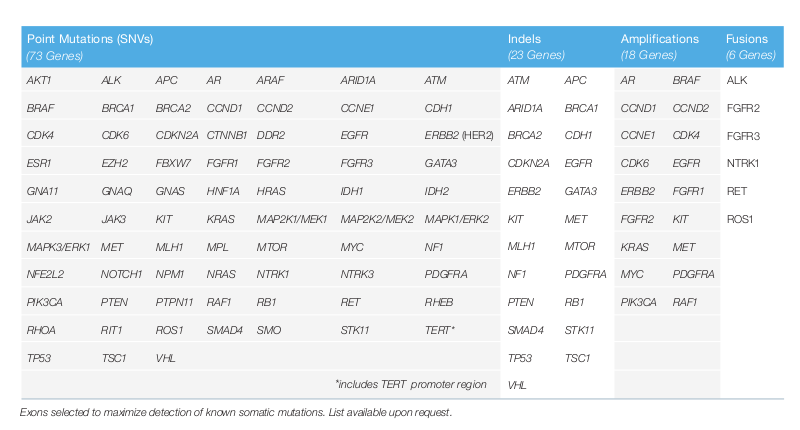
Available today Guardant360 the most advanced in its class among tumor genomic tests is used daily testing by clinicians with cancer patients. The Guardant360 test analyzes 74 genes in a blood sample to identify the presence of point tumor mutations rearrangements insertions and deletions indels beyond microsatellite instability. Guardant360 CDx is the first FDA-approved comprehensive liquid biopsy for all advanced solid tumors.
Guardant360 CDx offers patients and clinicians a simple faster blood test to help inform personalized treatment options. Thanks for the replies from everyone regarding their experience with the Guardant360 blood biopsy. Guardant360 CDx is a lab test that detects genetic mutations found in circulating cell-free DNA cfDNA to help doctors identify patients with non-small cell lung cancer who should be.
More than 40000 patients. This laboratory test is performed with a blood sample from cancer patients in an advanced stage stage III or IV in order to find an appropriate therapy option for a therapy with targeted drugs. Guardant360 is a liquid biopsy.
Help patients with advanced cancer obtain the right treatment. The format is GTR000000011 with a leading prefix GTR followed by 8 digits a period then 1 or more digits representing the version. When I was diagnosed with Stage IV lung cancer In November 2013 which was determined from the cancer cells found in my pleural effusion basic mutation testing EGFR BRAC KRAS ALK was done at that time and I did not have any of those mutations.
High Concordance with Tissue 1. Advantages and quality criteria of Guardant360 Guardant360 is the first clinically validated comprehensive liquid biopsy that is available and allows a comprehensive. The Guardant360 CDx FDA approval was based on.
Guardant360 검사를 소개합니다. The Guardant360 liquid biopsy testmay help if. The Guardant360 assay is a commercially available next-generationsequencing test for identifying alterations in 73 genes from cell-free tumor DNA and.
How Guardant Health is supporting cancer care during the pandemic. Moreover Guardant360 can be used for monitoring and aftercare of.
 Workflow For The Guardant360 Cell Free Circulating Dna Ngs Genomic Download Scientific Diagram
Workflow For The Guardant360 Cell Free Circulating Dna Ngs Genomic Download Scientific Diagram
 Guardant360 Guardant Health Amea
Guardant360 Guardant Health Amea
 Guardant360 Therapy Planning With Blood Liquid Biopsy Therapyselect
Guardant360 Therapy Planning With Blood Liquid Biopsy Therapyselect
![]() Solutions Guardant360 Guardant Health Amea
Solutions Guardant360 Guardant Health Amea
Genomic Testing With Guardant360
 Guardant Health Looks Forward To The Liquid Biopsy Opportunity In 2019 Nasdaq Gh Seeking Alpha
Guardant Health Looks Forward To The Liquid Biopsy Opportunity In 2019 Nasdaq Gh Seeking Alpha
 Study Shows Guardant360 Liquid Biopsy Predicts Response To Pembrolizumab Based Immunotherapy In Patients With Metastatic Non Small Cell Lung Cancer Business Wire
Study Shows Guardant360 Liquid Biopsy Predicts Response To Pembrolizumab Based Immunotherapy In Patients With Metastatic Non Small Cell Lung Cancer Business Wire
 Guardant360 Therapy Planning With Blood Liquid Biopsy Therapyselect
Guardant360 Therapy Planning With Blood Liquid Biopsy Therapyselect
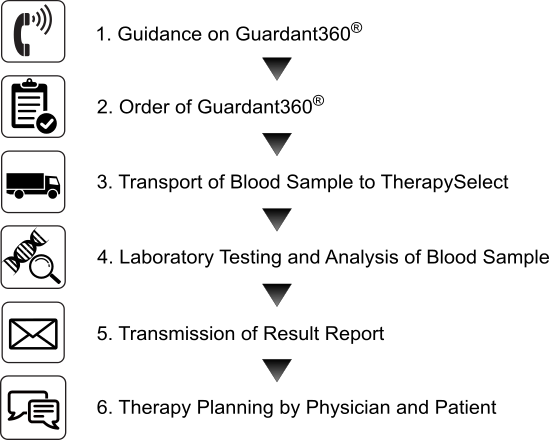 Guardant360 Process And Order Therapyselect
Guardant360 Process And Order Therapyselect
 Guardant360 Therapy Planning With Blood Liquid Biopsy Therapyselect
Guardant360 Therapy Planning With Blood Liquid Biopsy Therapyselect


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.