A WCD can give a shock to your heart to make it. How is the LifeVest WCD different from an automatic external defibrillator AED.
 Wearable Cardioverter Defibrillator Sudden Cardiac Arrest Foundation
Wearable Cardioverter Defibrillator Sudden Cardiac Arrest Foundation
LifeVest should only be removed when a patient is showering or bathing.

Wearable external defibrillator. Wearable Cardio Defibrillator and Automatic External Defibrillator Description. An arrhythmia may cause your heart to suddenly stop beating effectively. The LifeVest was approved by the FDA in 2001 and has been prescribed more than 35000 times according to ZOLL which is based in Pittsburgh and part of the Asahi Kasei Group Company.
It is lightweight and easy to wear. A wearable cardioverter defibrillator eg LifeVest is considered experimental investigational or unproven for any other indication. The trade name of the WCD 2000 System was changed to LifeVest in 2002.
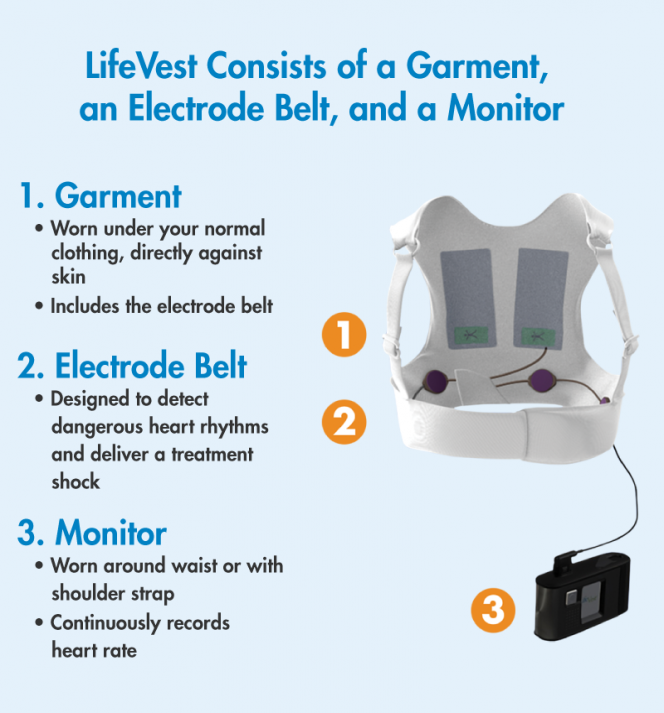
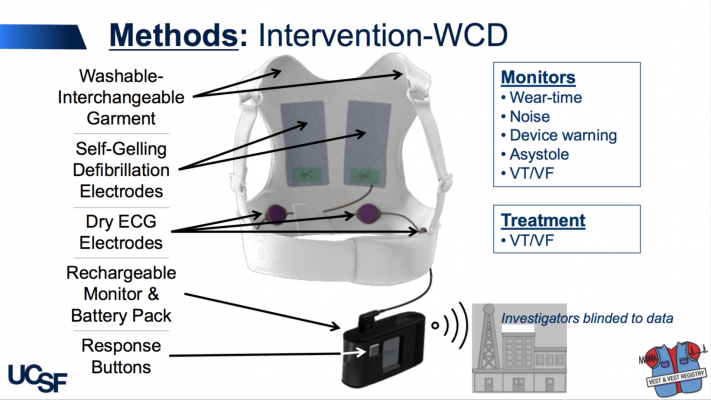
The LifeVest wearable cardioverter defibrillator WCD is designed to protect patients at risk of sudden cardiac death SCD when a patients condition is changing and permanent SCD risk has not been established. Use of an AED requires bystander assistance. A Wearable Cardioverter Defibrillator WCD.
Jeden Tag werden zehntausende Menschen durch LifeVest vor dem PHT geschützt. An external defibrillator is attached in the wearable cardioverter defibrillator to a vest that is worn by the patient. This document addresses the wearable cardioverter defibrillator an external vest-like garment device that is intended to perform the same tasks as an implantable cardioverter defibrillator ICD without requiring any invasive procedures.
An arrhythmia is an irregular heart rate. The LifeVest is a microprocessor-based and programmable. This is the story of the LifeVest the only FDA-approved wearable cardioverter defibrillator WCD indicated to reduce the risk of sudden cardiac death SCD in patients with reduced left ventricular ejection fraction LVEF who have suffered an.
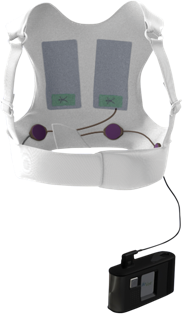
Automated external defibrillators are generally either kept where health professionals and first responders can use them health facilities and ambulances as well as public access units which can be found in public places including corporate and government offices shopping centres restaurants public transport and any other location where people may congregate. Learn more about this lifesaving therapy. While some defibrillator devices are implanted under the skin the LifeVest WCD is worn directly against the patients skin.
Patients should wear the LifeVest WCD at all times including while sleeping. Der tragbare Defibrillator LifeVest gibt Patienten die gefährdet sind einen plötzlichen Herztod PHT zu erleiden ein beruhigendes Gefühl und die lebensrettende Behandlung im Ernstfall. An automatic external defibrillator AED is primarily considered a safety device kept in the home as a precautionary measure to address a possible acute event rather than a device needed for active treatment.
The LifeVest wearable cardioverter defibrillator is a proven therapy for patients at risk of sudden cardiac death. The aim of this study was to describe usage of the wearable cardioverter-defibrillator WCD during mandated waiting periods following myocardial infarction MI for patients perceived to be at high risk for sudden cardiac arrest SCA. The vest contains electrodes or pads that can shock your heart if the monitor detects a life-threatening arrhythmia.
The wearable cardioverterdefibrillator may protect against sudden death during the immediate period after myocardial infarction before ICD implantation is. An AED in the home is therefore not. A WCD is a vest with a small monitor.
What is a wearable cardioverter defibrillator WCD. Current device guidelines and insurance coverage require waiting periods of either 40 days or 3 months before implanting a. It is highly used in patients to.
 Wearable Cardioverter Defibrillator Thoracic Key
Wearable Cardioverter Defibrillator Thoracic Key
 Wearable Defibrillator Cuts Overall Mortality But Not Sudden Deaths After Heart Attack Daic
Wearable Defibrillator Cuts Overall Mortality But Not Sudden Deaths After Heart Attack Daic
 Lifevest Wearable Defibrillator Reduces Total Mortality By 36 Percent At 90 Days
Lifevest Wearable Defibrillator Reduces Total Mortality By 36 Percent At 90 Days
 Zoll Lifevest Wearable Defibrillator Get Quote Rfq Price Or Buy
Zoll Lifevest Wearable Defibrillator Get Quote Rfq Price Or Buy
 Wearable Automatic External Defibrillator Radiology Case Radiopaedia Org
Wearable Automatic External Defibrillator Radiology Case Radiopaedia Org
 Zoll Receives Fda Clearance For New Lifevest Model Daic
Zoll Receives Fda Clearance For New Lifevest Model Daic
 Fda Approves Zoll S Wearable Defibrillator For Pediatric Use Rt
Fda Approves Zoll S Wearable Defibrillator For Pediatric Use Rt
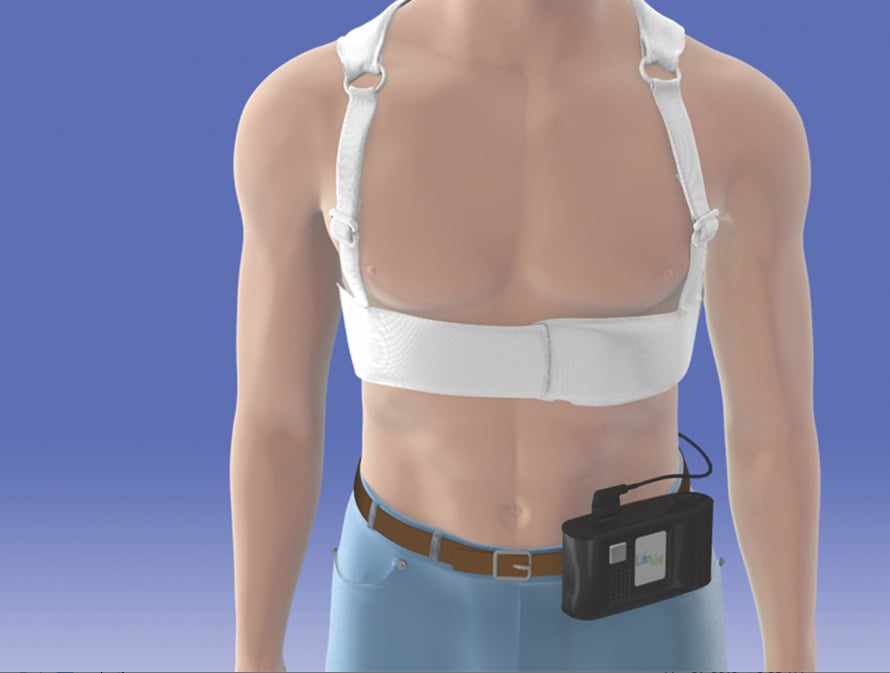 Wearable Defibrillator Offers A Bridge Therapy Daic
Wearable Defibrillator Offers A Bridge Therapy Daic

 Wearable Defibrillator Is Said To Save Lives But Patients And Doctors Question At What Cost
Wearable Defibrillator Is Said To Save Lives But Patients And Doctors Question At What Cost
 Wearable Cardioverter Defibrillator A Life Vest Till The Life Boat Icd Arrives Sciencedirect
Wearable Cardioverter Defibrillator A Life Vest Till The Life Boat Icd Arrives Sciencedirect
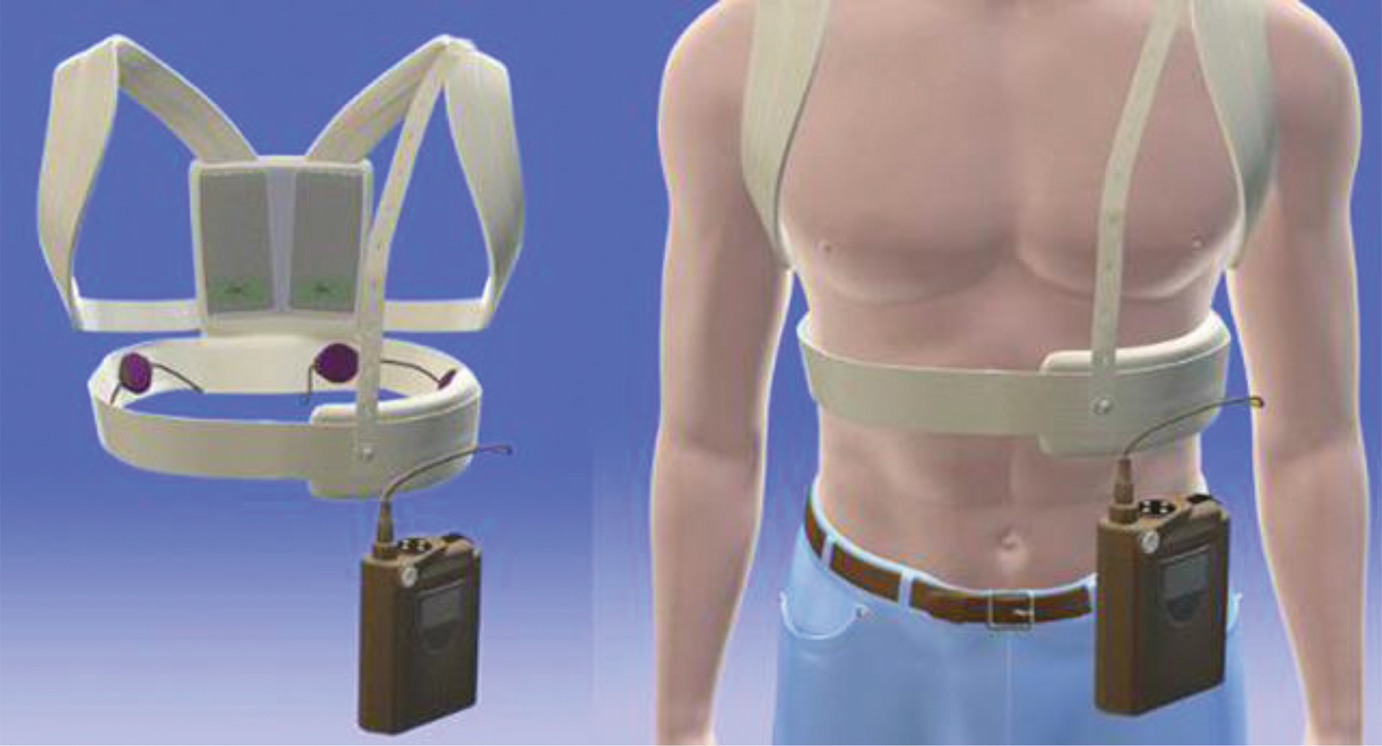 Wearable Cardioverter Defibrillators Springerlink
Wearable Cardioverter Defibrillators Springerlink
 Wearable Cardioverter Defibrillators Circulation
Wearable Cardioverter Defibrillators Circulation
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.